The ability to manufacture devices at the very small, or micro, scale brings with it interesting applications possibilities and surprising challenges. For instance, certain physical phenomena may not be generally encountered with doing macro scale experiments on the lab bench but are dominant when experiments are done with a micro scale “lab-on-a-chip.” The field of microfluidics deals with the behavior and manipulation of fluids, both liquids and gases, that are constraint to a small scale (1 to several hundred micrometer). The physical properties of fluids at this scale are particularly interesting and could possibly be exploited. For instance, fluids at a certain scale are no longer practically compressible. Also, it is difficult to maintain pressure or pressure-driven flow within a micro scale fluidic system. One example is the phenomenon of back-flow whereby fluid moves in one direction when pressure is applied only to have it reverse and flow back when the pressure is released. Due to the physics of scaling laws, these various non-intuitive phenomena are less severe when transporting gasses than liquids in a micro scale device. For instance, gravity has no influence on gasses and diffusion of gasses at the micro scale is VERY fast. Therefore, gas species can mix through diffusion with simple laminar flows at low Reynolds numbers.
In nature microscale liquid and gas flow is quite common. Maybe engineers should look at insects to see how fluids can most effectively be moved around at the microscale. The insect’s respiratory system, for instance, uses microscale flow transport to achieve an absolutely essential physiological function. The respiratory system at first glance seems so simple – just some gas-filled tubes. At the same time it seems so foreign – no breathing through mouth or nose, no lungs or blood or hemoglobin involved (at least not at first glance). The respiratory system is also elegant in its simplicity through ingenious feedback loops – fine-tuning through direct nervous innervation or by sensing chemical compositions of the blood (called hemolymph in insects).
This system that is key to maintaining homeostasis is also as varied as insects are varied. Ingenious adaptations are seen in insects that live in different habitats – within soil, in water, in arid climates, in cold climates, within a host, while pupating, while flying, while walking, etc.
In this post I’ll focus mostly on the “generalized” terrestrial insect.
The insect’s tracheal system
The insect’s respiratory system’s main functions are to carry oxygen and carbon dioxide to and from the tissues. The gas-filled tubes, called tracheae, start at the spiracles, which are paired openings in the cuticle that can occur in almost every or only a few segments (depending on the insect species). The spiracles are innervated by the nervous system and can be open and closed through muscular action.

Spiracles of a lepidopteran larva. Two are visible in this picture; they appear as black openings surrounded by yellow ring. (Picture by Geoff Gallice)
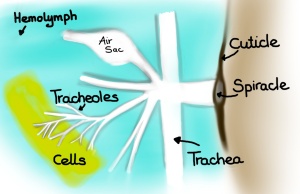
Inside the insect the tracheal systems starts out as two or more longitudinal trunks, which further into the insect branch into smaller and smaller channels. Tracheae range in diameter from a few mm to 1 μm. The smallest branches are called tracheoles, which range from 1 to 0.1 μm in diameter. The tracheoles’ tips are in contact with cells and are often fluid-filled. This means that at the tips of the tracheoles oxygen has to cross first a liquid, then the tracheolar walls, then cross the plasma membrane of the cell and finally move through the cytoplasm of the cells to reach the mitochondria. Carbon dioxide makes the same trek, but in the opposite direction.
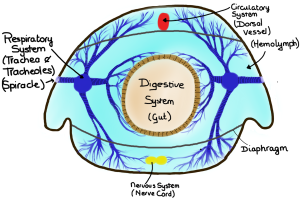
Cross-section of the tracheal system of “general” insect. Other insects may have more than 2 longitudinal trunks and/or air sacs and/or no spiracle functional in a particular segment. (Drawing by Marianne Alleyne)

Cross-section of tracheal tube. Shown is an epidermal outer layer bathing in hemolymph, an inner intima layer, and taenidia, which reinforce the intima layer (much like a car’s radiator hose is reinforced by coil of steel). (Drawing by Marianne Alleyne)
In order to get oxygen to move into the cells and carbon dioxide to move out of the body, all while limiting energy use, insects rely on diffusion and convection.
- Diffusion: passive movement of molecules down their concentration gradient.
Oxygen diffusion rate is more rapid in air than in liquid, and because the tracheal system consists mostly of air-filled tubes the gas-exchange by diffusion is extremely rapid and quite substantial. While the cells use the oxygen, the partial pressure of oxygen decreases near the tips of the tracheoles, which then draws in more oxygen from the tracheae and the air outside of the insect. Diffusion is of critical importance at the tips of the tracheoles, but it can only work at micrometer distances since at this point the oxygen has to move through a liquid. This is why almost every cell in the insect is at close proximity to a tracheole tip. The cells that require high respiratory rates (flight muscle cells, for instance) may even have multiple tracheole tips invaginating the cell wall.
The prominent Danish physiologist August Krogh devised an early model (1920) of grasshopper respiration that was based on oxygen partial pressures and suggested that simple diffusion could provide adequate gas exchange in insects. This claim had a huge impact on the field early and quickly became dogma, despite some contemporary contrary evidence. In 1964 “a next-generation Dane” Torkel Weis-Fogh showed that in larger or more active insects the distance between the spiracles and the tips of the tracheoles was too great and that diffusion was insufficient if the insect was active. So what other mechanisms do insects use to move oxygen and carbon dioxide through the tracheae?
- Convection: bulk movement of a gas or a liquid driven by differential pressures.
Compared to diffusion, convection can achieve greater gas exchange rates over longer distances. The trade-off is that it requires more work and thus more energy.
- One type of convective air-movement seen in insects is, confusingly, termed suction ventilation. This movement of air is primarily achieved by opening and closing, or shielding differentially, a subset or all of spiracles at different parts of the body. This creates a pressure difference between the spiracles and the tracheal ends and thus creates passive airflow.
- Autoventilation is achieved by using wings or legs to pump air through the body.
- Abdominal pumping, using the muscles in the abdomen to pump air.
- Another, more important, type of convective air movement is achieved by the tracheae (tracheal compression), and their associated air sacs (tracheal sections that are not reinforced by taenidia), collapsing and inflating.

Digital image of silkworm Bombyx mori trachea with taenidea (100X mag) by Paul Joseph, Birmingham, UK.
With novel imaging and measurement techniques researchers have shown that rhythmic tracheal compression is quite common. Synchrotron x-ray imaging has been used since 2003 to visualize tracheal structures in living insects. We can now see that muscles compress the exoskeleton of the insect, which results in air moving through the tracheae since the tracheae and air sacs are collapsing. These studies, which are now linked with real-time flow-through respirometry, show that rapid cycles of collapse and re-inflation in a variety of insects’ tracheal systems – especially in the head and thorax regions – results in large changes in tracheal volume and are major components of ventilation in insects. Even the different ways the tracheal system can compress and re-inflate is probably as varied as insects themselves.
Must watch: Beetle Tracheae Collapsing (video 1, video 2, video 3). Supporting materials from Westneat, et al. (2003) Science V299 (5606), DOI: 10.1126/science.1078008
Using the insect’s tracheal system as inspiration for microscale flow devices
The impetus for this blog post was a recent paper I came across entitled: Selective pumping in a network: insect-style microscale flow transport, by Yasser Aboelkassem and Anne E. Staples. The topic of microscale flow transport is of interest to engineers because there are many applications that need precise flow control of just microliters of gas or fluid. Microscale flow devices or microfluidic devices are already around us and are assumed to change research tools in the life sciences, aid drug discovery and chemical analyses, and revolutionize health diagnostics. Modern DNA sequencing systems (Fluidigm, Illumina, Roche (454), etc.) rely on microfluidics. “Droplet” analyses also rely on microfluidic technologies. Cheap paper-based microfluidic immunoassay tests will become important for healthcare in the developing world (Diagnostics for All). And those are just a few of the applications.
Insect tracheal systems have been of particular interest to engineers now that we are able to visualize this physiological process (moving fluid and air through tubes) in action. Insects are able to move fluid and air with precision with minimal energy requirements and little input from outside of the body. All characteristics that would work well within a “lab-on-a-chip”.
Insects use contractions at very specific places along the tracheal system, which probably affords them more precise control than if just relying on the more crude pressure difference approach. Through these small, but targeted, compressions the airflow to specific areas of the body can be achieved. This is apparently not an easy task to achieve in small-scale devices, and one can appreciate that by imagining larger tubes.
Imagine a garden hose. By stepping onto the hose at one point the fluid or air inside the hose will be displaced to either side. As soon as you step off the hose the fluid or air will rush back (=back-flow). How can one create directional flow of a particular speed or pressure? By stepping at multiple spots along the hose, sometimes closing the hose off completely and other times only partially. Stepping off of the hose according to a certain sequence will also result in a directional flow. Of course, this is just one hose. Now imagine doing this with hoses that are branching into thinner hoses, that have multiple openings, with many more feet that can step on and off of them…and then think of a miniaturized version of that and you will see the insect’s tracheal system.
This is what Aboelkassem and Staples tried to model in their paper. The fluid dynamics research group headed by Staples at Virginia Tech’s Department of Engineering Science and Mechanics is apparently closely aligned with the biomechanics research lab of Jake Socha at the same departments. The Socha lab is one of the labs that has used synchrotron x-ray imaging to study insect respiration. The theoretical and computational model proposed by Aboelkassem and Staples incorporates rhythmic wall contractions along a network of tubes (in this case an 8-armed network) without the need for valves. The model’s tubes/channels are squeezed at different places along the tube. The contractions were actuated to move with different time lags from each other. In this way the researchers showed that fluids can be transported and flow velocity, pressure and direction can be controlled.
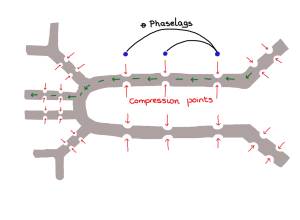
Simplified insect tracheal network modeled by Aboelkassem & Staples. The “tracheae” can be collapsed at multiple points within the network (red arrows). The collapse motion protocol can be varied by including time phase lags. The result is precise movement of the liquid or gas (green arrows).
We have learned from insects that localized rhythmic tracheal contraction is an effective way to move gas through micro-tubes. Aboelkassem and Staples claim to have been inspired by the insect’s tracheal system to model “selective pumping in a network”. They show, by using theoretical (low Reynolds number) flow analyses and computation fluid dynamics, that the network might also enable fluids to be transported precisely into particular tubes without the use of mechanical valves – at least through an 8-armed tracheal system. It will be interesting to see if this research group will continue to follow the insects’ lead by adding more branches/channels, by adding air sacs (which seem to be very important in insects), by making the model 3D, by adding actively opening and closing ports/spiracles to the outside, and by incorporating the diversity of insect respiratory systems into different models. If done correctly, and by using microfabrication techniques that can make flexible micro- and nano-scale tubes, it is likely insects can make a contribution to the rapidly growing technological field of microfluidics.
Resources and References:
For a more detailed description of the insect’s respiratory system:
- Harrison, J. F., J. S. Waters, A. J. Cease, J. M. VandenBrooks, V. Callier, C. J. Klok, K. Shaffer and J. J. Socha. (2013) How Locusts Breathe. Physiology V28:(1) 18-27. DOI:10.1152/physiol.00043.2012
- Harrison, J.F. (2009) Respiratory System. Pages 889-895 in: “Encyclopedia of Insects, 2nd ed.”. Editors, V.H. Resh and R. Carde, Elsevier, San Diego.
- Harrison, J.F. (2009) Tracheal System. Pages 1011-1015 in: “Encyclopedia of Insects, 2nd ed.”. Editors, V.H. Resh and R. Carde, Elsevier, San Diego.
- Image Gallery: Butterfly Metamorphosis in 3D.
For description of how synchrotron x-ray imaging is used to study insect respiration:
- Westneat, M. W., O. Betz, R. W. Blob, K. Fezzaa, W. J. Cooper and W. -K. Lee. (2003) Tracheal respiration in insects visualized with synchrotron x-ray imaging. Science V299, 5606, pp. 558-560. DOI: 10.1126/science.1078008
- Westneat, M.W., J.J. Socha and W.-K. Lee. (2008) Advances in biological structure, function and physiology using synchrotron x-ray imaging. Annual Review of Physiology V70: 119-142. DOI: 10.1146/annurev.physiol.70.113006.100434
- Socha, J.J., W.-K. Lee, J.F. Harrison, J.S. Waters*, Fezzaa, K. and M.W. Westneat. (2008) Correlated patterns of tracheal compression and convective gas exchange in a carabid beetle. Journal of Experimental Biology V211: 3409-3420. DOI: 10.1242/jeb.019877
- Socha, J.J., T. Förster and K.J. Greenlee. (2010) Issues of convection in insect respiration: Insights from synchrotron x-ray imaging and beyond. Respiratory Physiology and Neurobiology V173S S65–S73. DOI: 10.1016/j.resp.2010.03.013
- Waters J.S.,W.-K. Lee, M.W. Westneat and J. J. Socha. (2013) Dynamics of tracheal compression in the horned passalus beetle. American Journal of Physiology:Regulatory, Integrative and Comparative Physiology V304(8) R621-7. DOI: 10.1152/ajpregu.00500.2012
Bioinspired microscale flow structures:
- Chu, A. K-H. (2004) Transport control within a microtube. Physical Review E V70 061901 DOI: 10.1103/PhysRevE.70.061902
- Aboelkassem, Y. and A. E. Staples. (2013) Selective pumping in a network: insect-style microscale flow transport. Bioinspiration & Biomimetics V8(2) 026004. DOI: 10.1088/1748-3182/8/2/02600
Special thanks to Dr. Paul Kenis for taking the time to discuss microscale flow transport with me. I appreciate his patience with me – clearly a novice. Bedankt!



Pingback: Expiscor (1 July 2013) | Arthropod Ecology
Pingback: Morsels for the mind – 5/7/2013 › Six Incredible Things Before Breakfast
Pingback: The insect cuticle: (1) multi-functionality |
Very nice, job!!!! You may find this paper interesting too: Webster, M. R., De Vita, R., Twigg, J. N., Socha, J. J., Mechanical Properties of Tracheal Tubes in the American Cockroach (Periplaneta Americana), Smart Materials and Structures, 20:094017, 2011.
Raffaella, thank you very much for reading the post. I did read a lot of the Socha-work in preparation for this post and that particular one is excellent.
I encourage anyone interested in this topic to look into publications from the Socha-lab at Virginia Tech – just watch out for “falling” snakes. http://www2.esm.vt.edu/~jjsocha/socha_lab/Publications.html
Thank you again for reading the post and commenting with useful information for other readers. I very much appreciate it.
M
Pingback: Let me introduce you to our Bioinspiration symposium at ICE